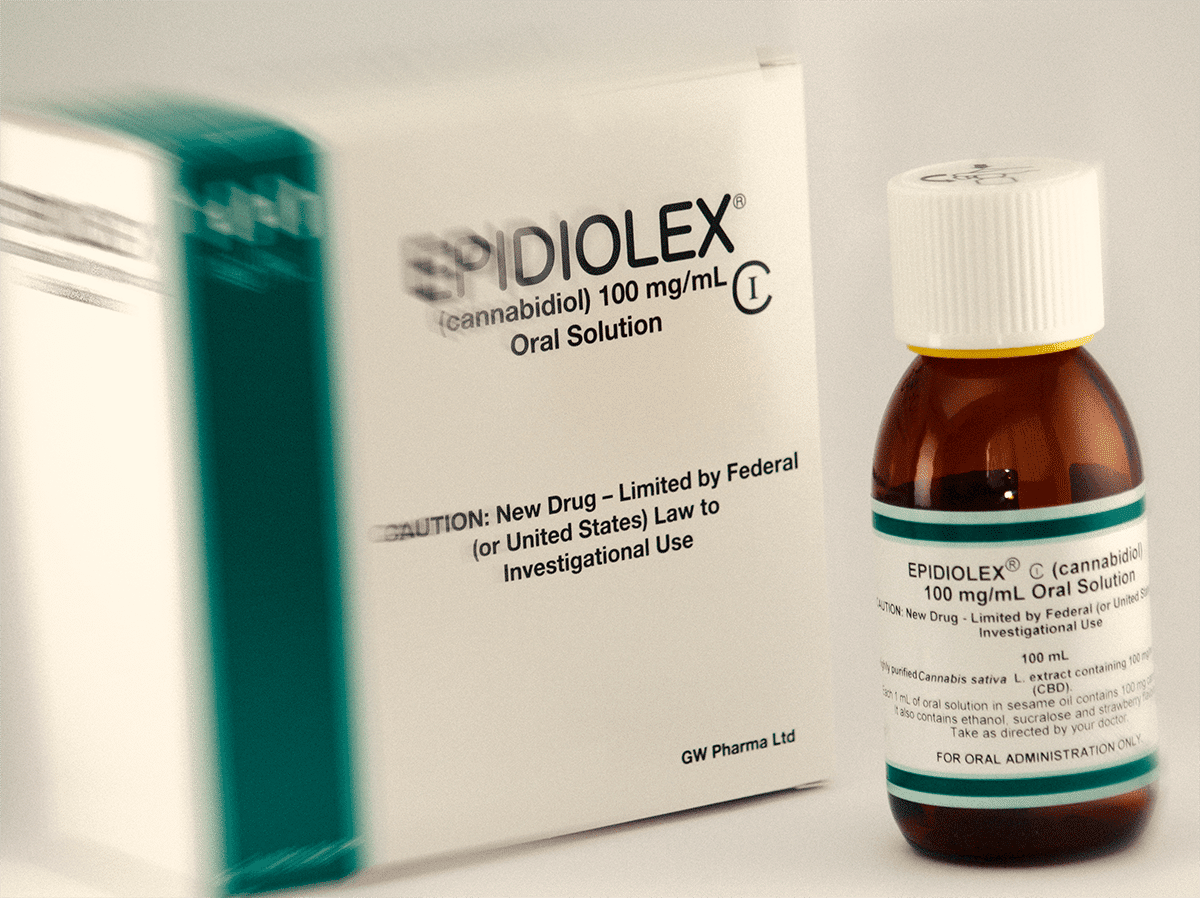One of the biggest headlines in the marijuana space has been that cannabidiol has been proven to help slow seizures in children and young adults. The fact that cannabidiol doesn’t contain THC, which is where the “high” comes from, is what makes it so promising.
Two large clinical trials have just released results in the last two months and cannabidiol was shown to help reduce seizures in two forms of epilepsy, the hardest and most rare kinds, Dravet Syndrome, and Lennonx-Gastaut Syndrome.
In the first trial, the study had 225 young people who have Lennox-Gastaut Syndrome. They were split into groups and given either a higher or lower dose of the drug or a placebo for 14 weeks. The results showed that the higher dose group participants saw a 42% drop in their seizure occurrence. The lower does saw a 37% drop and the placebo group saw a 17% drop.
The second trial had 120 children with Dravet syndrome where half were given the drug and half received a placebo. In the half that received the drug, forty-three percent of participants saw their seizures reduced by half, and 5% stopped having seizures entirely. The placebo group barely saw a change.
A new drug could soon be approved to treat seizures and it’s a syrup called Epidiolex. It could be the very first cannabidiol-based drug that the FDA approves.
Giant drugmaker GW Pharma is now able to apply for designation as the company was able to show that the product addresses a critical need. This could speed up the approval process.
GW Pharma expects to submit its new drug application to the FDA by the middle of the year.