By Asa Waldstein
This is the second article in a three-part Compliance Essentials for Hemp-CBD Companies series. I explore commonly overlooked manufacturing, labeling, and marketing essentials that ingestible cannabinoid companies need to be compliant. This second segment focuses on labeling.
With a lack of clarity happening at the federal level, hemp cannabinoid companies should act like they want to be regulated. Although the federal government has an unfavorable position on hemp cannabinoids, it is best to follow dietary supplement labeling regulations, in my opinion.
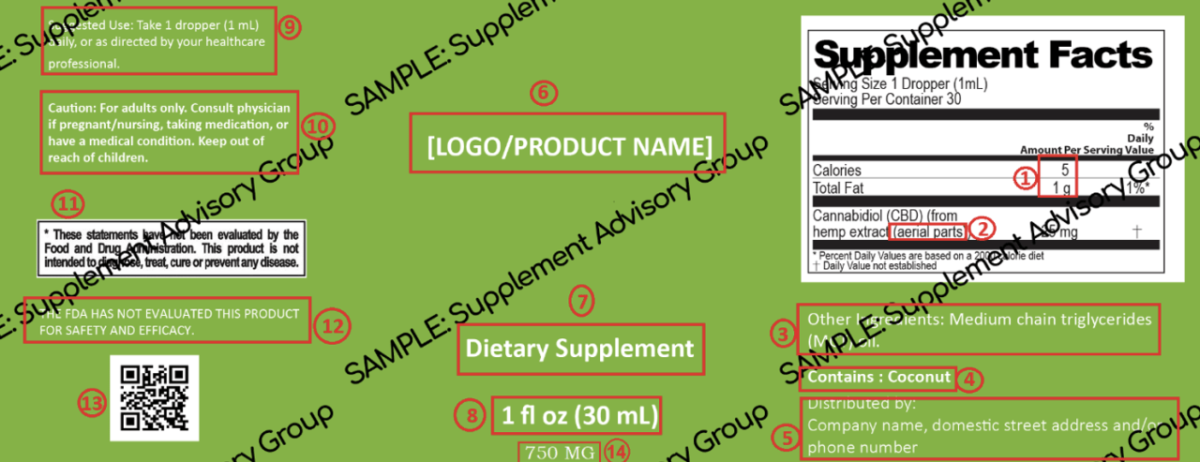
Here is an example of a label layout.
This sample label should not be used without final legal or regulatory approval.
Here are some essentials for Hemp-CBD labels (see numbers on the label).
- Proper rounding is important, and this makes the FDA happy and demonstrates that a company knows the basics of a fact panel. For example, four calories are rounded to 5 calories. Here is an excellent labeling resource from the American Herbal Products Association (AHPA).
- Plant parts are required in the fact panel.
- Other Ingredients should be listed under the fact panel.
- Common allergen disclosure and placement are essential. Common allergen disclosure is required, and lack of proper disclosure is the basis for numerous product recalls.
- Domestic street address or phone number. This is a commonly overlooked but essential part of a supplement label. This is required for Adverse Event Reporting, a core aspect of cGMPs. This is how supplement companies track customer complaints and inform the FDA of serious adverse events such as hospitalization. It should be located under the Other Ingredients section.
- Product name
- Statement of identity is required in the Principal Display Panel (PDP).
- The net quantity of contents should be on the bottom 30% of the label.
- Suggest Use or Serving Size should be located be in the left panel.
- Cautions or Warnings are product specific, and several states require a warning for pregnancy, nursing, and children. In general, this may satisfy most state warning requirements except for Prop 65 and additional California’s AB 45 (see number 12).
- DSHEA disclaimer is required on supplements with structure-function claims. Many states require this on CBD labels even if no claims are present.
- Additional warning is required in states such as California.
- QR Code linking to a third-party COA is required by most states. For fun, follow this QR Code to see where it takes you.
- Total amount of cannabinoids is required to register products in states like West Virginia. This is a strange rule but it is written state regulations and is essential for successful registration.
Supplement Facts or Something Else? Are consumable hemp products a supplement, a food, or a made-up new category such as “Products Facts?”
This is an ongoing debate, so my personal and non-legal opinion is that the FDA regulates based on the intended use. I believe it is ethical and accurate to label ingestible hemp products as supplements, and we discuss this here.
Not long ago, Louisiana did not allow the use of the word “supplement” in a “Supplement Facts Panel” on hemp products sold in their state but instead required a “Product Facts Panel.” This did the industry a disservice, and thankfully Louisiana has now retracted this rule. However, they oddly do not allow the word “dietary” (as in dietary supplement) on products sold in their state, and this is likely the reason so many products are labeled as “hemp supplements.”
Label Control: Labels should be tightly controlled in a manufacturing facility, and labels should be locked up and not “hanging out.” Preventing accidental product adulteration is a critical part of cGMPs and not having proper label control is a great way to increase the chances of mix-ups. I have seen cringy things such as employees printing out labels and applying them to bottles without quality oversight. An extreme example of this is the lawsuit against Curaleaf for mixing up CBD and THC labels. This reportedly resulted in a wrongful death case, trips to the emergency room, and widely reported recalls.
- The FDA requires tight label control. Having uncontrolled labels in the warehouse and office is a great way to irritate an FDA investigator, resulting in a more stringent FDA inspection. This is one of the tips we talk about in this Getting Ready For FDA webinar.
Supplement Fact Panel’s listed in the incorrect format are not likely to attract a warning letter but are a clear sign to the authorities of potential manufacturing issues. In the eyes of the FDA, if a company doesn’t know the rules of a proper label layout, this could indicate deeper manufacturing concerns. It is better to have the layout correct, so please verify label nuances such as font size are correct.
There is so much more to being a compliant hemp-CBD company. Here are the free webinars cGMP Basics for Hemp-CBD Companies and Distributor Requirements for Hemp-CBD Companies to support your compliant growth.
Disclaimer: These are for informational purposes only and are not intended to replace competent legal or regulatory advice. Have a qualified attorney or consultant look over your labels for compliance.
Asa Waldstein is a 20-year dietary supplement executive now focusing on bridging the compliance, marketing, and regulatory gap between the supplement and hemp industries. Asa also is the principal of the consulting company Supplement Advisory Group, a boutique group focusing on marketing risk analysis and practical marketing solutions for the web and social media. Waldstein is chair of the American Herbal Products Association’s (AHPA) Cannabis Committee, and his Regulatory Education Series platform regularly hosts free events for the community. Learn more and contact at AsaWaldstein.com.

The post Compliance Essentials for Hemp-CBD Companies, Part 2 of 3: Labeling first appeared on Let’s Talk Hemp.