To read parts one and two of this three part series, visit:
Part 1: https://www.letstalkhemp.com/compliance-essentials/
Part 2: https://www.letstalkhemp.com/compliance-essentials-2/
By Asa Waldstein
This is the final article in a three-part Compliance Essentials for Hemp-CBD Companies series. I explore commonly overlooked manufacturing, labeling, and marketing essentials that ingestible cannabinoid companies need to be compliant. This third segment focuses on marketing, which is my favorite compliance aspect. Marketing compliance and reading enforcement trends are essential to any regulatory affairs professional, executive, investor, marketing manager, social media supervisor, and sales representative. I love helping companies communicate their marketing messages in a compliant and effective way. This is the core reason I started my consulting company and free Regulatory Education Series
With a lack of clarity at the federal level, hemp cannabinoid companies should act like they want to be regulated. Although the federal government has an unfavorable position on hemp cannabinoids, it is best to follow dietary supplement marketing regulations, in my opinion. The guardrails of what constitutes a claim in online marketing seem to be evermoving, which is one of the important reasons to follow FDA warning letters. I write a weekly post called Warning Letter Wednesday, where notable enforcement activity and trends are discussed. Join the free LinkedIn group here.
Most warning letters and agency actions occur because of online marketing statements. This article discusses common mistakes and best practices for online marketing.
Here are some essentials for Hemp-CBD marketers.
What Can I Say About My Products?
- What is a Claim: Learning what not to say is the best way to help develop a compliant marketing strategy. Here is a What is a Claim video on this topic.
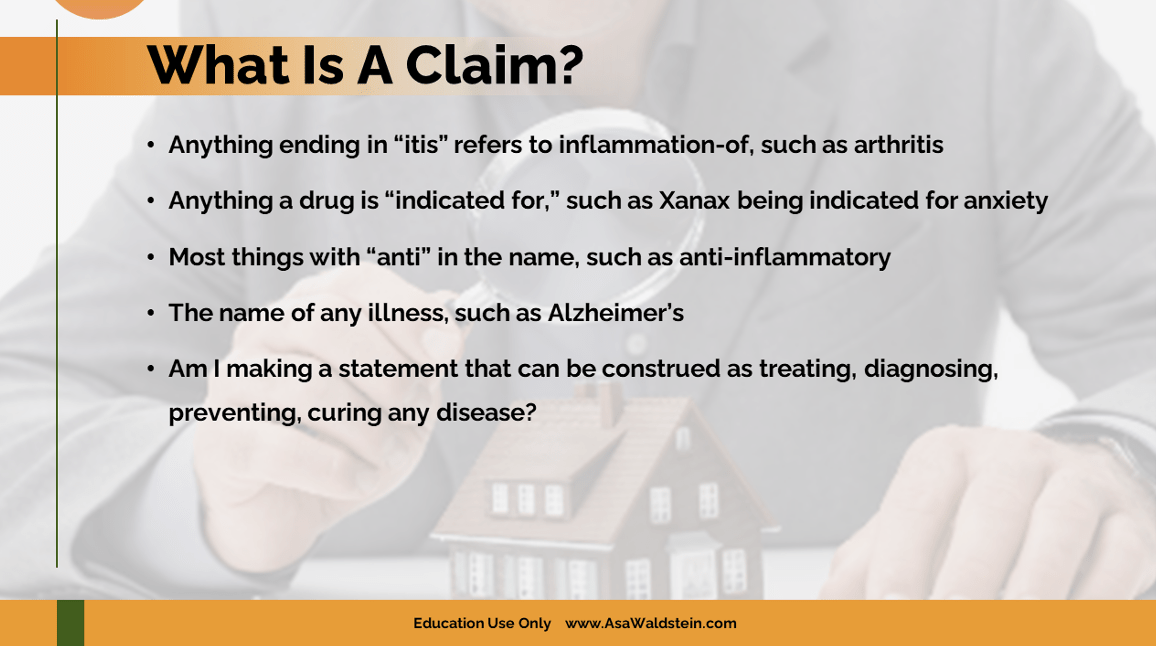
- Quality of Life Statements: Nothing in the hemp cannabinoid world is without risk. In general, making truthful quality of life statements such as soothe, calm, and relax is a lower risk. Nearly every CBD warning letter includes high-risk online claims. It is easy for companies to cross the line from making quality of life statements into disease or drug claims. Here are a few examples.
- Helps you wake up feeling refreshed = a lower risk quality of life statement
versus
Helps with your insomnia = a high-risk disease claim - Supports relaxation = a lower risk quality of life statement
versus
Help anxiety = a high-risk disease claim
- Helps you wake up feeling refreshed = a lower risk quality of life statement
All Marketing is “Labeling”: We wouldn’t write a disease word such as depression on a product label, but this same word may slip by in a social media post, hashtag, or blog. This is how warning letters occur. I find it helpful to think of all marketing as an extension of the label. Here is a video I made for marketers and copywriters on this topic.
Find and Replace High-Risk Words: A vital part of marketing compliance is replacing high-risk words with lower-risk and truthful alternatives. The FDA/FTC can easily find high-risk words. Here is a post about this.

Blogs: These are an extension of the “label” and, therefore, should be compliant. If a blog is located on a website, it is easy to cross from “education” to marketing. Several recent warning letters have referenced claims made in blogs, and this is an enforcement trend to watch. The best strategy is not to have high-risk words in blogs, including study citations. Here is a post about general rules for companies that must use educational blogs on a company website.
Social Media: Many warning letters contain claims made on social media. Here are some areas to be aware of.
- Hashtags are helpful customer attraction tools, but they are also considered marketing claims. Here is a video about this.
- Old social media posts: The FDA reviews social media posts that are several years old, and they look at an old post in the same manner as a current one. Here is a detailed post about this.
- Reposting: Posting links such as clinical studies and videos can be considered marketing.
- Infographics
- “Liking” a post: This is considered to substantiation the “claim” on a social media wall.
Videos are Marketing Statements: Claims made in videos on platforms such as YouTube are becoming more common in warning letters. Just a few years ago, citations to videos’ claims were nearly nonexistent, but now the FDA has tools to search videos for high-risk disease words quickly.
Lower Risk Marketing Example: Here are two examples of lower-risk ways to talk about a product or showcase a cause the company believes in.

Flying Below The FDA’s Radar
It is a misconception to think that they are “not on FDA’s radar because companies are small.” If a company is selling products online, they are “fair game” for enforcement, and therefore all pieces of marketing should be compliant. This is because the agencies likely use keyword search programs that can find certain word combinations such as CBD+anxiety+depression on any website. Once a disease or drug claim is found, authorities will keep digging and expanding the review to include social media, which is mentioned in nearly every warning letter. In one letter, a claim made on a YouTube video was cited. When I looked at this video, it had only 57 views, demonstrating that even small marketing pieces can attract attention.
There is so much more to being a compliant hemp-CBD company. Here are some free resources. FTC’s Advertising Guide, FTC’s Disclosure for Social Media Influencers, and cannabis attorney Nathalie Bougenies’s Above The Law articles.
Disclaimer: These are for informational purposes only and are not intended to replace competent legal or regulatory advice. Have a qualified attorney or consultant review your marketing for compliance.
# # #
Asa Waldstein is a 20-year dietary supplement executive now focusing on bridging the compliance, marketing, and regulatory gap between the supplement and hemp industries. Asa also is the principal of the consulting company Supplement Advisory Group, a boutique group focusing on marketing risk analysis and practical marketing solutions for the web and social media. Waldstein is chair of the American Herbal Products Association’s (AHPA) Cannabis Committee, and his Regulatory Education Series platform regularly hosts free events for the community. Learn more and contact at AsaWaldstein.com.
The post Compliance Essentials for Hemp-CBD Companies, Part 3 of 3: Marketing first appeared on Let’s Talk Hemp.




